
Exhibit 99.1

Sonoma Pharmaceuticals, Inc. Investor Presentation April 2025
| 1 |

Legal Disclaimers or INVESTOR PRESENTATION This communication is for informational purposes only. The information contained herein does not purport to be all - inclusive. The data contained herein is derived from various internal and external sources. No representation is made as to the reasonableness of the assumptions made within or the accuracy or completeness of any information contained herein. Any data on past performance is no indication as to future performance. Sonoma Pharmaceuticals, Inc. and its subsidiaries (“Sonoma” or, the “Company”) assumes no obligation to update the information in this communication. This presentation is not an offer to buy or the solicitation of an offer to sell Sonoma securities. FORWARD - LOOKING STATEMENTS Except for historical information herein, matters set forth in this presentation are forward - looking within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including statements about the commercial and technology progress and future financial performance of Sonoma Pharmaceuticals, Inc. and its subsidiaries. These forward - looking statements are identified by the use of words such as “believe,” “achieve,” and “expect,” among others. Forward - looking statements in this presentation are subject to certain risks and uncertainties inherent in the Company’s business that could cause actual results to vary, including such risks that regulatory clinical and guideline developments may change, scientific data may not be sufficient to meet regulatory standards or receipt of required regulatory clearances or approvals, clinical results may not be replicated in actual patient settings, the Company will not have sufficient capital to implement its business plan, invalidated or circumvented by its competitors, the available market for the Company’s products will not be as large as expected, the Company’s products will not be able to penetrate one or more targeted markets, revenues will not be sufficient to fund further development and clinical studies, uncertainties relative to fluctuations in foreign currency exchange rates, global economic conditions, prospective tariffs or changes to trade policies, as well as uncertainties relative to varying product formulations and a multitude of diverse regulatory and marketing requirements in different countries and municipalities, and other risks detailed from time to time in the Company’s filings with the Securities and Exchange Commission. The Company disclaims any obligation to update these forward - looking statements, except as required by law. TRADEMARKS AND INTELLECTUAL PROPERTY All trademarks, service marks, and trade names of the Company and its subsidiaries or affiliates used herein are trademarks, service marks, registered trademarks of the Company as noted herein. Any other product, company names, or logos mentioned herein are the trademarks and/or intellectual property of their respective owners. 2
| 2 |

About Sonoma Sonoma Pharmaceuticals is a global healthcare leader for developing and producing stabilized hypochlorous acid (HOCl) products for a wide range of applications, including wound, eye, oral and nasal care, dermatological conditions, podiatry, animal health care and non - toxic disinfectants. Sonoma’s products are clinically proven to reduce itch, pain, scarring, and irritation safely and without damaging healthy tissue. In - vitro and clinical studies of HOCl show it to safely manage skin abrasions, lacerations, minor irritations, cuts, and intact skin. Sonoma’s products are sold either directly or via partners in 55 countries worldwide. Sonoma actively seeks new distribution partners. 3
| 3 |
Hypochlorous Acid Industry Leader Hypochlorous Acid Industry Leader 22 U.S. FDA clearances as 510(k) medical devices, transition to MDR completed for all revenue - producing products, and extensive worldwide regulatory clearances Utilized on millions of patients worldwide without a single report of serious adverse effect Focused on billion - dollar markets in Rx and OTC dermatology, wound care, eye, oral and nasal care, podiatry, animal health and non - toxic disinfectants, treating common injuries and managing irritations Diverse Global Healthcare Business Diverse Global Healthcare Business Investment Highlights Over 20 years of experience developing and manufacturing products containing HOCl, over 100 research articles, papers and clinical and case studies, over 50 active U . S . and international patents, and continual product innovation Robust and diverse international partner network selling into over 55 countries and extensive worldwide regulatory clearances means products can be commercialized faster FDA registered medical device manufacturing capabilities in Mexico lowers COGS and creates opportunities for volume plays 4
| 4 |
Sonoma’s Microcyn ® Technology Patented, Stabilized Triple - Action Topical Technology • HOCl kills microbes including bacteria, fungi and viruses while assisting healing • Relieves pain and itch • Improves wound care by cleaning and moistening the wound and peri - wound area • Safely manage skin abrasions, lacerations, minor irritations, cuts, burns, and intact skin • Applications in dermatology include atopic dermatitis, scar treatment and daily care May replace other treatments with side effects or health concerns • Overused antibiotics may cause deadly epidemics such as MRSA • May replace steroids for some patients • Avoids harmful ingredients such as benzoyl peroxide Cost Effective • Reduces hospital/physician visits • Medicare/hospital savings from reduced hospital stays 5
| 5 |
Sonoma’s Microcyn ® Technology Unparalleled Safety • No drug - to - drug interaction or contraindications • Millions of patients treated worldwide without a single report of serious adverse effect • 30+ human clinical trials with over 1,500 patients • Products contain all - natural hypochlorous acid Industry Leader • Strong U.S. and international regulatory portfolio • Longer shelf life than most competitors while retaining efficacy • Available in solution, hydrogels and in combination with silicone for scar treatment • White label available with custom packaging and design • Established Microcyn ® brand • State of the art manufacturing 6
| 6 |

Over 100 research articles and case and clinical studies showcasing both the efficacy and safety of our Microcyn ® technology • Dermodacyn® Disinfecting solution study on viability of SARS - CoV - 2, see https:// www.sciencedirect.com/science/article/pii/S019567012030339X • Endocyn® study on cellular toxicity, see https:// www.sciencedirect.com/science/article/abs/pii/S0099239917310439 • HOCl study evaluating virucidal activity in Vero E6 cells against SARS - CoV - 2, University of Barcelona, 11/27/2020 • Hypochlorous acid gel technology — Its impact on post - procedure treatment and scar prevention, see https://sonomapharma.com/wp - content/uploads/2020/08/Gold_et_al - 2017 - Journal_of_Cosmetic_Dermatology - 003.pdf • Topical stabilized hypochlorous acid: The future gold standard for wound care and scar management in dermatologic and plastic surgery procedures, see https://sonomapharma.com/wp - content/uploads/2020/08/Topical - Stabilized - HOCL - consensus - article - 2020.pdf • HOCl Article: Optimizing Wound Healing for Procedures – Apr 2018, see https://sonomapharma.com/wp - content/uploads/2018/11/Pracitcal - Derm - HOCl - article - 4 - 7 - 18 - wounds.pdf 7
| 7 |
Wide Range of Applications Wound Care Dermatology Eye Care Oral and Dental Care Animal Health Care Surface Disinfectants Podiatry 8 Senior Care
| 8 |

Our Wound Care Business is Growing in the U.S. and Internationally • Celebrating 20 years of selling wound care • In August 2024, we added Medline Industries, LP as a U.S. distributor for our wound care products – Medline is one of the largest medical supply distributors in the United States • In October 2024, we expanded our partnership with Medline to include distribution in Canada, and OTC wound care sales to retailers in both countries • We sell wound care across Europe and added a new distributor for wound care in Ukraine in 2024 • Expanding Product Lines: including new options for our Microcyn® Negative Pressure Wound Therapy Solution product line. • Product innovation: In June 2023, we announced a new application of our Microcyn® technology for intraoperative pulse lavage irrigation treatment . 9
| 9 |

Industry - Leading Safe and Effective Dermatology Products • Products to relieve symptoms of atopic dermatitis, reduce scarring, and for daily skin care • Line of office dispense products targeting growing med spa market • Eczema seal of approval • All natural ingredients • No harmful chemicals • OTC, office dispense, and Rx products available • Innovating packaging available including high end and cost competitive options • Continual Product Innovation : Introduced Lumacyn TM Clarifying Mist in 2024, a daily toner formulated to reduce redness and manage blemishes by reducing infections. 10
| 10 |
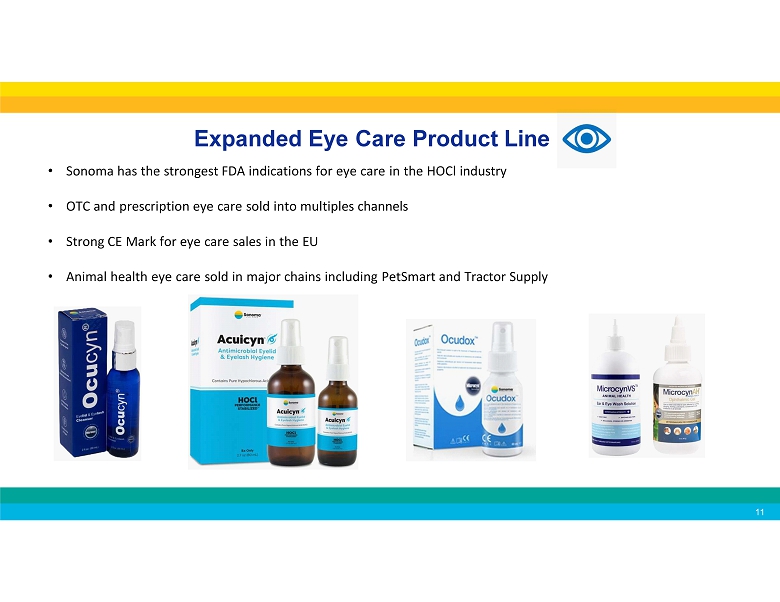
Expanded Eye Care Product Line • Sonoma has the strongest FDA indications for eye care in the HOCl industry • OTC and prescription eye care sold into multiples channels • Strong CE Mark for eye care sales in the EU • Animal health eye care sold in major chains including PetSmart and Tractor Supply 11
| 11 |

Robust U.S. and International Distribution Network OVER 40 GLOBAL PARTNERS AND GROWING We continue to: • Expand our presence in new markets by replicating what works in existing markets • Add new distribution partners and grow existing relationships • Co - develop innovative new products with partners • Work with partners to seek new approvals and certifications 12
| 12 |

• 57,153 square foot state - of - the - art facility • ISO 9001, ISO 13485 and cGMP certified • MOH, KFDA, SFDA, KSA, TGA, EN, Biocide and numerous other national listings and approvals • Shipping to over 55 countries, with multiple methods for shipments • Highly trained staff of 162 employees • Additional 11,840 square feet of overflow capacity CUTTING EDGE MANUFACTURING Flexible operations capable of CUSTOM LABELING, SIZING, PACKAGING & DISPENSE MODES High or small volume, large or small batch, delivered in 12 weeks Currently operating at 40% capacity with margin of approximately 35% 13
| 13 |

14
| 14 |
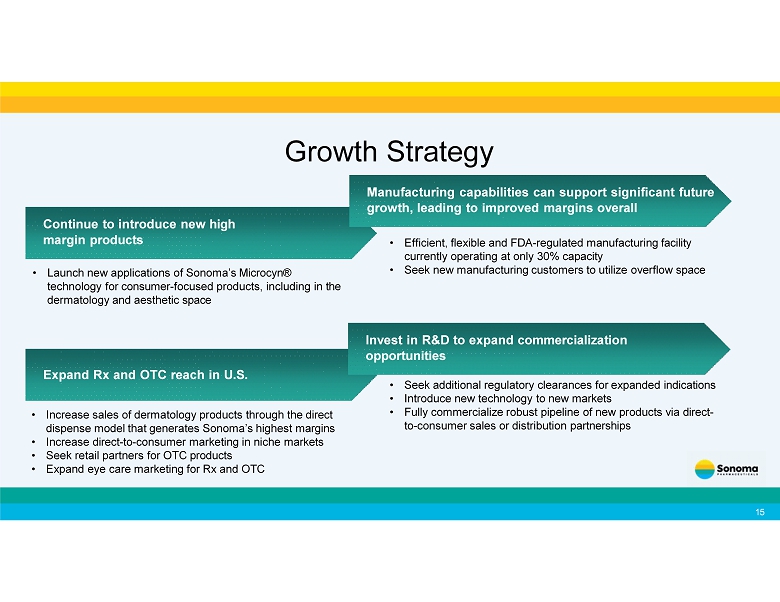
Continue to introduce new high margin products Manufacturing capabilities can support significant future growth, leading to improved margins overall • Launch new applications of Sonoma’s Microcyn® technology for consumer - focused products, including in the dermatology and aesthetic space Growth Strategy • Efficient, flexible and FDA - regulated manufacturing facility currently operating at only 30% capacity • Seek new manufacturing customers to utilize overflow space Expand Rx and OTC reach in U.S. • Increase sales of dermatology products through the direct dispense model that generates Sonoma’s highest margins • Increase direct - to - consumer marketing in niche markets • Seek retail partners for OTC products • Expand eye care marketing for Rx and OTC 15 Invest in R&D to expand commercialization opportunities • Seek additional regulatory clearances for expanded indications • Introduce new technology to new markets • Fully commercialize robust pipeline of new products via direct - to - consumer sales or distribution partnerships
| 15 |

New Market Opportunities Sonoma is expanding its partnerships and product offerings in the eye care, all - natural skin care, medical spa and animal health care industries. • The global eye care market size was estimated at USD $70.78 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.72% from 2024 to 2030. 1 • The global natural skin care products market size was valued at $6.7 billion in 2021 and is expected to expand at a CAGR of 6.6% from 2022 to 2030. 2 • The global medical spa market size was estimated at USD 18.6 billion in 2023 and is expected to grow at a CAGR of 15.13% from 2024 to 2030. 3 o North America dominated the medical spa market with a share of 41.1% in 2023, due to higher expenditure on wellness tourism than other regions. 4 • The global animal health market size was valued at USD 62.40 billion in 2023 and is projected to grow at a CAGR of 9.0% from 2024 to 2030. 5 1. Grand View Research, Eye Care Market Report, 2024 - 2030 , available at https:// www.grandviewresearch.com/industry - analysis/eye - care - market - report 2. Grand View Research, Natural Skin Care Products Market Report, 2022 - 2030 , available at https:// www.grandviewresearch.com/industry - analysis/natural - skin - care - products - market 3. Grand View Research, Medical Spa Market Size, Share & Growth Analysis Report 2024 - 2030 , available at https:// www.grandviewresearch.com/industry - analysis/medical - spa - market 4. Ibid. 5. Grand View Research, Animal Health Market Size & Share Report, 2024 - 2030 , available at https:// www.grandviewresearch.com/industry - analysis/animal - health - market 16
| 16 |

Introduction of new products using our Microcyn ® technology 17 • In December 2024, we announced the relaunch of our prescription eye care product, Acuicyn ® , our prescription dermatology products Celacyn ® , Levicyn ® and Epicyn ® , and our over - the - counter Lasercyn ® Dermal Spray and Lasercyn Gel . • In September 2024, we announced the consumer - friendly redesign of Ocucyn ® Eyelid & Eyelash Cleanser . • In April 2024, we announced expansion of our Microcyn ® Negative Pressure Wound Therapy Solution product line, now available in 250mL, 450mL and 990mL sizes to meet the diverse needs of healthcare professionals and patients. • In January 2024, we launched Lumacyn TM Clarifying Mist , a new direct - to - consumer skincare product in the United States. Lumacyn is an all - natural daily toner formulated with Microcyn technology to soothe the skin and relieve irritation. • In June 2023, we announced a new application of our Microcyn technology for intraoperative pulse lavage irrigation treatment , which can replace commonly used IV bags in a variety of surgical procedures. • Our MicrocynAH ® products are now available through Pets at Home , with over 450 stores across the UK, through our partner Compana Pet Brands • In May 2024, we announced expansion of our MicrocynAH animal health care products in the Menards ® chain of home improvement stores in the United States, through Compana Pet Brands . • In April 2023, we launched Podiacyn TM Advanced Everyday Foot Care direct to consumers for over - the - counter use in the United States. Recent Business Developments
| 17 |

Expanding commercialization opportunities by investing in R&D • We successfully transitioned all of our commercialized products in Europe, including eye care , wound care , scar gel , acne products and atopic dermatitis products to the new European Union (EU) Medical Device Regulation (MDR) . • Our manufacturing facility and five of our products are successfully registered with the Medicines & Healthcare products Regulatory Agency (MHRA) in the United Kingdom, including our wound irrigation solution and hydrogel, scar management products and acne products . • In 2024 we received two new 510(k) clearances from the FDA, including specific over - the - counter indications for the face , eyelid and eyelashes , as well as improved biocompatibility for our Microcyn - based solution and hydrogel. • In March 2023 , we announced new EPA claims for Nanocyn® Hospital - Grade Disinfectant for effective use against MRSA , Salmonella , Norovirus , Poliovirus , and as a fungicide . Nanocyn was previously approved for use against COVID - 19 and emerging pathogens including Ebola virus , Mpox , and SARS - CoV - 2 . Nanocyn also received the esteemed Green Seal ® Certification . In August 2024 , the Australian TGA approved new claims for use against C . auris and C . diff . TM • Reliefacyn® Advanced Itch - Burn - Rash - Pain Relief Hydrogel was awarded the N ATIONAL E CZEMA A SSOCIATION S EAL OF A CCEPTANCE • A recent publication in the journal Neurourology and Urodynamics highlighted the potential for Microdox® in the management of urinary tract infections, or UTIs, in children with neurogenic or non - neurogenic bladder dysfunction. 1 1. Singh G - K, Deshpande A, Schlegel G, Starkey M, Taghavi K. The rationale for bladder washouts in children with neurogenic bladder. Neurourol Urodyn. 2024;1 - 6. doi:10.1002/nau.25450 Recent Business Developments 18
| 18 |

Continuously expanding our distributor network 19 • In April 2025, we announced the launch our of acne products in the U.K. through a leading health and beauty retailer and pharmacy chain. • In January 2025, we partnered with WellSpring Pharmaceutical Corporation for the sale of our Microcyn technology - based products to large retailers in the United States . • In March 2025, we expanded our agreement with WellSpring to include additional consumer focused products. • In August 2024, we announced a new distribution agreement with Medline Industries, LP for the marketing and distribution of our wound care products in the United States . • In October 2024, we expanded our agreement with Medline for marketing and distribution of our wound care products in Canada , and the sale of OTC wound care products to retailers in both countries. • In July 2024, we announced a new distribution agreement for wound care in Ukraine . • In April 2023, our partner Te Arai BioFarma Limited launched BabySoothe for diaper rash applications in Taiwan . • In April 2024, Te Arai launched Microdacyn for wound care in Taiwan . • Our partner Microderm Technologies recently launched Dermodacyn for wound care applications in Thailand . • Our partner Brill Pharma SL is now selling Sonoma’s eye care products in Italy , Spain , Portugal and Germany . • In January 2023, we entered into a distribution agreement with Daewoong Pharmaceutical Co., Ltd. , one of the largest pharmaceutical companies in South Korea , for marketing and distribution of Primocyn TM Skin Solution products Recent Business Developments
| 19 |

Management Amy Trombly CEO Amy Trombly is 20 our Chief Executive Officer and also serves on our Board of She counseled public Directors. companies corporate for over two decades in and securities law and mergers and acquisitions, including as the owner and manager of Trombly Business Law, PC . In her earlier career, Ms . Trombly was a Vice President at State Street Bank and Special Counsel at the U . S . Securities and Exchange Commission . Ms . Trombly is a member of the bar in Massachusetts and Colorado . Bruce Thornton COO Bruce Thornton has served as our Chief Operating Officer, Vice President of Global Operations, and US General Manager since 2004 , and currently as Executive Vice President and Chief Operating Officer . He served as Vice President of Operations for Jomed (formerly EndoSonic Corp.) from January 1999 to September 2003, and as Vice President of Manufacturing for Volcano Therapeutics, an international medical device company, following its acquisition of Jomed, until March 2004 . Mr . Thornton received a B . S . in Aeronautical Science from Embry - Riddle Aeronautical University and an M . B . A . from National University . He also has served in the US Army . Jerry Dvonch CFO Mr . Dvonch serves as our Chief Financial Officer . He joined us from the SpineCenter Atlanta where he was the controller and Senior Vice President of Finance and Accounting since March 2017 . From March 2016 to April 2016 he was a consultant controller for DS Healthcare Group, Inc . Prior to that he was the director for external reporting and director of finance of NeoGenomics Laboratories from July 2015. He has over experience with SEC 2005 to July 10 years of reporting. Mr. Dvonch is a licensed Certified Public Accountant in New York. He holds a Master of Business Administration in Finance from the University of Rochester and a Bachelor of Business Administration in Accounting from Niagara University.
| 20 |

Key Financial Metrics 21 Nine months ended December 31, 2024 - » Revenues increased 13% compared to same period FY 2024 » Net loss improved $1.1 million , or 29% , compared to same period FY 2024 Three months ended December 31, 2024 - » Revenues increased 14% compared to same period in FY 2024 » Positive cash flow from operations for the quarter with $5.2 million of cash at December 31, 2024
| 21 |

APPENDIX: Product Portfolio
| 22 |

23
| 23 |

24
| 24 |

U.S. Animal Health Care MicrocynAH ® family of advanced animal healthcare products, safe to use on pets and livestock, and perfect for hot spots, pink eye, scratches, skin rashes and ulcers, cuts, burns, post - surgical sites, irritated skin and lacerations. • Sold in national pet - store retail chains and specialty stores such as PetSmart , Chewy , Tractor Supply , Cabela’s , Bass Pro Shops and Menards . MicrocynVS ® veterinarian - strength animal care for use in vet clinics and animal hospitals. 25
| 25 |
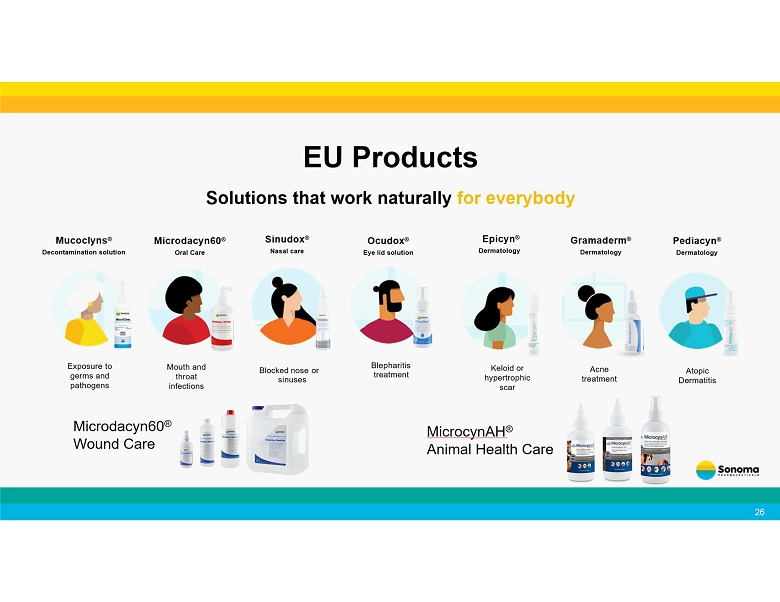
26
| 26 |

27
| 27 |

Middle East 28
| 28 |

Singapore, Malaysia, Thailand, Indonesia Wound Care South Korea BioDerm Biodacyn60 Wound Care Hong Kong Microdacyn60 Oral Care China Microcyn The Philippines Microdacyn MicrocynAH ® Asia 29
| 29 |

Gramacyn Kit for Acne Skin Latin America T Epicyn reat Scars Right from the Start Australia & New Zealand 30
| 30 |

THANK YOU Sonoma Pharmaceuticals Inc. 5445 Conestoga Court, Suite 150 Boulder, CO 80301 United States of America Phone: 1 - (800) 759.9305 Email: busdev@sonomapharma.com Website: www.sonomapharma.com NASDAQ: SNOA
| 31 |