
Exhibit 99.1

Sonoma Pharmaceuticals, Inc. NASDAQ: SNOA March 2021

Legal Disclaimers INVESTOR PRESENTATION This communication is for informational purposes only. The information contained herein does not purport to be all - inclusive. T he data contained herein is derived from various internal and external sources. No representation is made as to the reasonableness of the assum pti ons made within or the accuracy or completeness of any information contained herein. Any data on past performance is no indication as to future performance. Sonoma Pharmaceuticals, Inc. (“Sonoma” or, the “Company”) assumes no obligation to update the information in thi s communication. This presentation is not an offer to buy or the solicitation of an offer to sell Sonoma securities. FORWARD - LOOKING STATEMENTS Except for historical information herein, matters set forth in this presentation are forward - looking within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including statements about the commercial and technology pro gress and future financial performance of Sonoma Pharmaceuticals, Inc. and its subsidiaries (the “Company”). These forward - looking statements are identified by the use of words such as “believe,” “achieve,” and “strive,” among othe rs. Forward - looking statements in this presentation are subject to certain risks and uncertainties inherent in the Company’s business that could cau se actual results to vary, including such risks that regulatory clinical and guideline developments may change, scientific data may not be suff ici ent to meet regulatory standards or receipt of required regulatory clearances or approvals, clinical results may not be replicated in act ual patient settings, the Company will not have sufficient capital to implement its business plan, invalidated or circumvented by its competitors, the ava ilable market for the Company’s products will not be as large as expected, the Company’s products will not be able to penetrate one or more tar get ed markets, revenues will not be sufficient to fund further development and clinical studies, as well as uncertainties relative to varyin g p roduct formulations and a multitude of diverse regulatory and marketing requirements in different countries and municipalities, and other risks d eta iled from time to time in the Company’s filings with the Securities and Exchange Commission. The Company disclaims any obligation to update the se forward - looking statements, except as required by law.

Legal Disclaimers NON - GAAP FINANCIAL MEASURES Non - GAAP income (loss) from operations minus non - cash expenses (EBITDAS) is a non - GAAP financial measure. The Company defines operating income (loss) minus non - cash expenses as GAAP reported operating income (loss) minus operating depreciation and amorti zation, and operating stock - based compensation. The Company uses this measure for the purpose of modifying the operating income (loss) t o reflect direct cash related transactions during the measurement period. TRADEMARKS AND INTELLECTUAL PROPERTY All trademarks, service marks, and trade names of the Company and its subsidiaries or affiliates used herein are trademarks, se rvice marks, or registered trademarks of the Company as noted herein. Any other product, company names, or logos mentioned herein are the tra dem arks and/or intellectual property of their respective owners.

About Sonoma Sonoma Pharmaceuticals is a global healthcare leader for developing and producing stabilized hypochlorous acid ( HOCl ) products for a wide range of applications, including wound care, animal health care, eye care, nasal care, oral care and dermatological conditions. The company’s products reduce infections, itch, pain, scarring and harmful inflammatory responses in a safe and effective manner. In - vitro and clinical studies of HOCl show it to have impressive antipruritic, antimicrobial, antiviral and anti - inflammatory properties. Sonoma’s stabilized HOCl immediately relieves itch and pain, kills pathogens and breaks down biofilm, does not sting or irritate skin and oxygenates the cells in the area treated assisting the body in its natural healing process. Sonoma also manufactures disinfectants that are distributed outside of the U.S. and in certain countries where it has received regulatory clearance to state the disinfectant kills the coronavirus causing COVID - 19. The company’s products are sold either directly or via partners in 54 countries worldwide and the company actively seeks new distribution partners.

Investor Highlights Diverse global healthcare leader • Focused on billion - dollar Rx and OTC wound care, dermatology, eye, oral and nasal care, disinfectant use and animal health markets • Over 20 years of experience • Robust international partner network • Focused on expanding U.S. partner network • Strong manufacturing capabilities Unique, patented and FDA - cleared, CE and ISO approved Microcyn® Technology • HOCl is known to be among the safest and most effective ways to relieve itch, inflammation, and burns while stimulating natural healing through increased oxygenation and eliminating persistent microorganisms and biofilms • Proven antipruritic, antimicrobial, antiviral and anti - inflammatory properties • Disinfectant approved in Australia and Canada for use against COVID - 19 • Currently selling into 54 countries

Key Opportunities • Expand OTC reach in U.S. in the dermatology, eye, oral and nasal care, disinfectants and wound care markets • Fully commercialize existing products via distribution partners or directly • 21 U.S. FDA clearances as medical devices for Section 510(k) • CEs for over 39 products • Expand presence in the dental market • Continue to add international distribution partners; grow existing relationships • Robust product pipeline; continue to introduce new products worldwide • Continuous R&D efforts explore new product applications • Expand disinfectant sales globally

Recent Business Developments • Received new CE marking for Microdacyn 60 ® Eye Care in April 2020 • Entered into expanded distribution agreement with Brill International S.L. Spain for the eye care product and new territories Germany, Italy, Spain, Portugal and UK in May 2020 • Approval by ARTG in Australia to use Nanocyn ® Disinfectant and Sanitizer against SARS - CoV - 2 in May 2020 and in Canada in September 2020 • Nanocyn ® Disinfectant and Sanitizer passed material compatibility testing for Boeing and Airbus in a joint effort by Sonoma and MicroSafe Group • Launched nasal and oral care products in Australia and New Zealand with partner Te Arai BioFarma • Executed exclusive OTC distribution agreement with Crown Laboratories, Inc. on December 7, 2020 • Launched Endocyn ®, a HOCL - based biocompatible root canal irrigant that does not stain teeth or restorations, in December 2020 • Received EU Biocide listing in January 2021 from The European Chemicals Agency for our patented HOCl technology • Launched Microcyn ® OTC Wound and Skin Cleanser, our first direct to consumer over the counter product in February 2021
Sonoma’s Microcyn Technology • Stable triple - action topical technology • A powerful anti - microbial - reduces microbial load, including destruction of biofilms • Anti - inflammatory agent – reduces itch and pain • Anti - pruritic activity • Tissue healing (increased blood/oxygen flow to wound) • Unparalleled safety – No drug - to - drug interaction or contraindications • Millions of patients treated worldwide without single report of serious adverse effect • 30+ human clinical trials with over 1,500 patients • No mutations or resistance • Overused antibiotics may cause deadly epidemics such as MRSA • Cost effective • Preventative – reduces hospital/physician visits • Medicare/hospital savings – faster healing reduces hospital stays
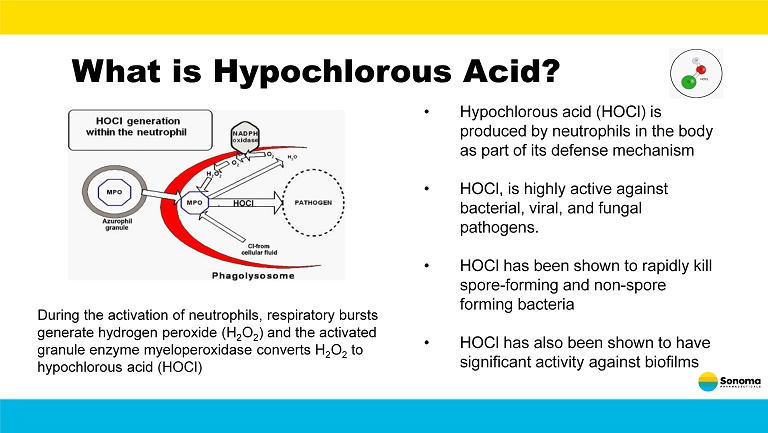
What is Hypochlorous Acid? During the activation of neutrophils, respiratory bursts generate hydrogen peroxide (H 2 O 2 ) and the activated granule enzyme myeloperoxidase converts H 2 O 2 to hypochlorous acid (HOCl) • Hypochlorous acid (HOCl) is produced by neutrophils in the body as part of its defense mechanism • HOCl, is highly active against bacterial, viral, and fungal pathogens. • HOCl has been shown to rapidly kill spore - forming and non - spore forming bacteria • HOCl has also been shown to have significant activity against biofilms
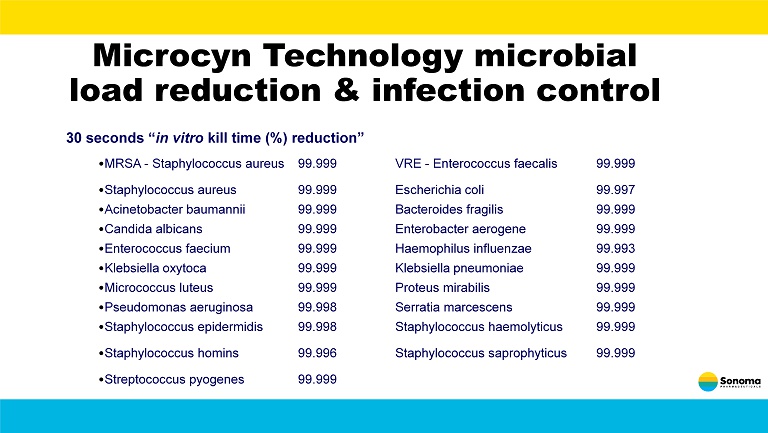
Microcyn Technology microbial load reduction & infection control 30 seconds “ in vitro kill time (%) reduction” • MRSA - Staphylococcus aureus 99.999 VRE - Enterococcus faecalis 99.999 • Staphylococcus aureus 99.999 Escherichia coli 99.997 • Acinetobacter baumannii 99.999 Bacteroides fragilis 99.999 • Candida albicans 99.999 Enterobacter aerogene 99.999 • Enterococcus faecium 99.999 Haemophilus influenzae 99.993 • Klebsiella oxytoca 99.999 Klebsiella pneumoniae 99.999 • Micrococcus luteus 99.999 Proteus mirabilis 99.999 • Pseudomonas aeruginosa 99.998 Serratia marcescens 99.999 • Staphylococcus epidermidis 99.998 Staphylococcus haemolyticus 99.999 • Staphylococcus homins 99.996 Staphylococcus saprophyticus 99.999 • Streptococcus pyogenes 99.999

Current Research and Development • Dermodacyn ® Disinfecting solution study on viability of SARS - CoV - 2, see https://www.sciencedirect.com/science/article/pii/S019567012030339X • Endocyn ® study on cellular toxicity, see https://www.sciencedirect.com/science/article/abs/pii/S0099239917310439 • HOCl study evaluating virucidal activity in Vero E6 cells against SARS - CoV - 2, University of Barcelona, 11/27/2020 • Hypochlorous acid gel technology — Its impact on post - procedure treatment and scar prevention, see https://sonomapharma.com/wp - content/uploads/2020/08/Gold_et_al - 2017 - Journal_of_Cosmetic_Dermatology - 003.pdf • Topical stabilized hypochlorous acid: The future gold standard for wound care and scar management in dermatologic and plastic surgery procedures, see https://sonomapharma.com/wp - content/uploads/2020/08/Topical - Stabilized - HOCL - consensus - article - 2020.pdf • HOCl Article: Optimizing Wound Healing for Procedures – Apr 2018, see https://sonomapharma.com/wp - content/uploads/2018/11/Pracitcal - Derm - HOCl - article - 4 - 7 - 18 - wounds.pdf

U.S. Summary • FDA - cleared products targeting: • atopic dermatitis, dermal lesions • scar management, • pain and itch relief and other dermatological conditions • eye - lash hygiene • acute and chronic wound care • oral care (dental surgery) • Ability to partner for OTC, Rx, and private labels • Relocating manufacturing to Mexico resulted in reduced COGs opening price - sensitive channels and opportunities for volume plays • Established and growing animal healthcare line of products • Change in business strategy has led to expanded U.S. business development targeting increasing relationships with U.S. distributors • Reduced focus on direct sales / Expanding into digital sales

Attractive U.S. Product Offerings
Robust International Distribution Network • Steadily growing existing partner base with broad indications • ISO/FDA registered/CE/EN/MOH/TGA registrations for state - of - the - art Mexico manufacturing facility • Ship to 54 countries globally • 40+ partners in dermatology, eye, wound, oral and nasal care, animal care and for surface disinfectant • Ability to produce over 400,000 high - quality units per month • Flexible operations capable of high/small volume, large/small batch, private label, and multiple configurations/packaging • Collaborative relationship with International Partners leading to product innovation and development

State of the Art Manufacturing Facility • ISO 9001 and 13485 certified • MOH, KFDA, SFDA, KSA, TGA, EN, Biocide and many other national listing/approvals • Shipping to 54 countries • First Rate Logistics • Highest Quality Standards • Flexibility of SKUs and packaging • Highly - trained staff

Europe

Latin America

Asia

Middle East

20 NASDAQ: SNOA Animal Health Care • MicrocynAH ® is a family of advanced animal healthcare products that are safe to use on all animals, no matter the species or age • Perfect for hot spots, scratches, skin rashes and ulcers, cuts, burns, post - surgical sites, irritated skin and lacerations. • We partner with MannaPro Products in the U.S. and Canada • Sold in national pet - store retail chains, farm animal specialty stores, at veterinarians, and mass retailers • Internationally, we partner with Petagon , Ltd. for the Asia and European sales

Sonoma Partnership Principles • Sonoma seeks long - term strategic partnerships, based on win/win business plans; 50/50 mentality with focus on what’s most important to each side. • Regular in - person and phone meetings to strengthen relationships. • “Do what you say and say what you do” mentality with a high standard of ethical behavior and transparent communication. No hidden agendas. • Sonoma seeks partners with innovative and differentiated technologies or products and strong distribution capabilities. Focus on profitable revenue growth consistent with current portfolio.
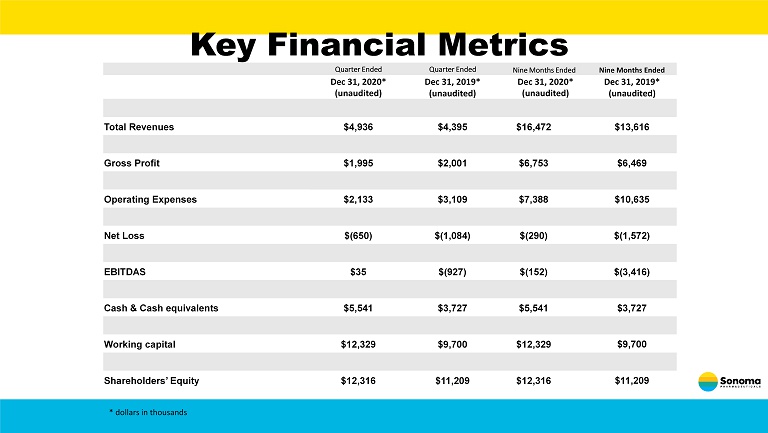
Key Financial Metrics Quarter Ended Quarter Ended Nine Months Ended Nine Months Ended Dec 31 , 2020* (unaudited) Dec 31, 2019* (unaudited) Dec 31, 2020* (unaudited) Dec 31, 2019* (unaudited) Total Revenues $4,936 $4,395 $16,472 $13,616 Gross Profit $1,995 $2,001 $6,753 $6,469 Operating Expenses $2,133 $3,109 $7,388 $10,635 Net Loss $(650) $(1,084) $(290) $(1,572) EBITDAS $35 $(927) $(152) $(3,416) Cash & Cash equivalents $5,541 $3,727 $5,541 $3,727 Working capital $12,329 $9,700 $12,329 $9,700 Shareholders’ Equity $12,316 $11,209 $12,316 $11,209 * dollars in thousands
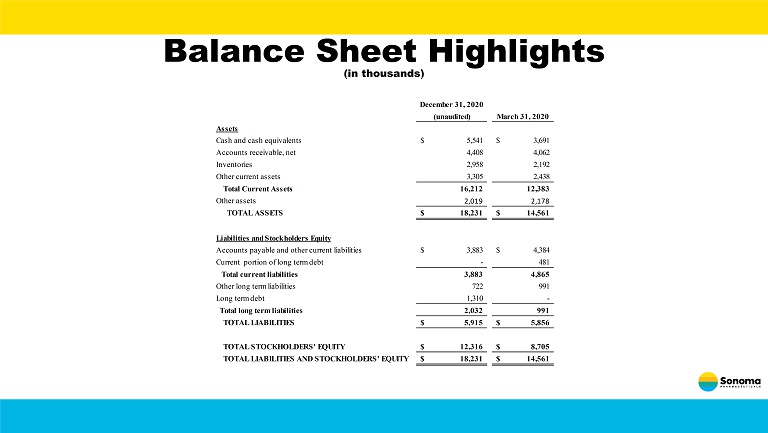
Balance Sheet Highlights (in thousands) December 31, 2020 (unaudited) March 31, 2020 Assets Cash and cash equivalents 5,541$ 3,691$ Accounts receivable, net 4,408 4,062 Inventories 2,958 2,192 Other current assets 3,305 2,438 Total Current Assets 16,212 12,383 Other assets 2,019 2,178 TOTAL ASSETS 18,231$ 14,561$ Liabilities and Stockholders Equity Accounts payable and other current liabilities 3,883$ 4,384$ Current portion of long term debt - 481 Total current liabilities 3,883 4,865 Other long term liabilities 722 991 Long term debt 1,310 - Total long term liabilities 2,032 991 TOTAL LIABILITIES 5,915$ 5,856$ TOTAL STOCKHOLDERS' EQUITY 12,316$ 8,705$ TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY 18,231$ 14,561$
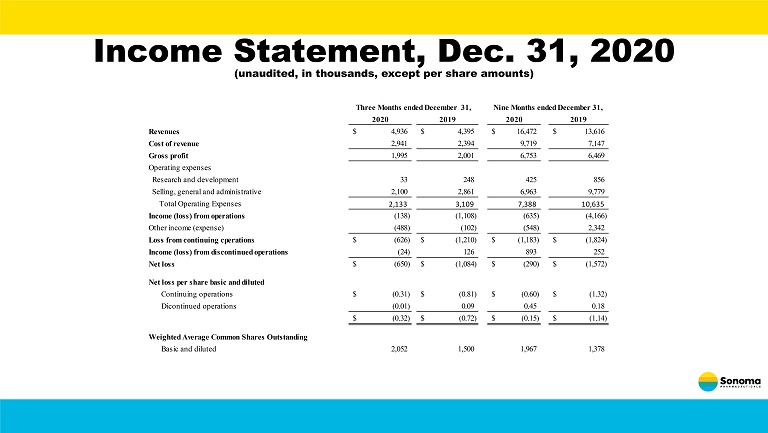
Income Statement, Dec. 31, 2020 (unaudited, in thousands, except per share amounts) 2020 2019 2020 2019 Revenues 4,936$ 4,395$ 16,472$ 13,616$ Cost of revenue 2,941 2,394 9,719 7,147 Gross profit 1,995 2,001 6,753 6,469 Operating expenses Research and development 33 248 425 856 Selling, general and administrative 2,100 2,861 6,963 9,779 Total Operating Expenses 2,133 3,109 7,388 10,635 Income (loss) from operations (138) (1,108) (635) (4,166) Other income (expense) (488) (102) (548) 2,342 Loss from continuing cperations (626)$ (1,210)$ (1,183)$ (1,824)$ Income (loss) from discontinued operations (24) 126 893 252 Net loss (650)$ (1,084)$ (290)$ (1,572)$ Net loss per share basic and diluted Continuing operations (0.31)$ (0.81)$ (0.60)$ (1.32)$ Dicontinued operations (0.01) 0.09 0.45 0.18 (0.32)$ (0.72)$ (0.15)$ (1.14)$ Weighted Average Common Shares Outstanding Basic and diluted 2,052 1,500 1,967 1,378 Three Months ended December 31, Nine Months ended December 31,
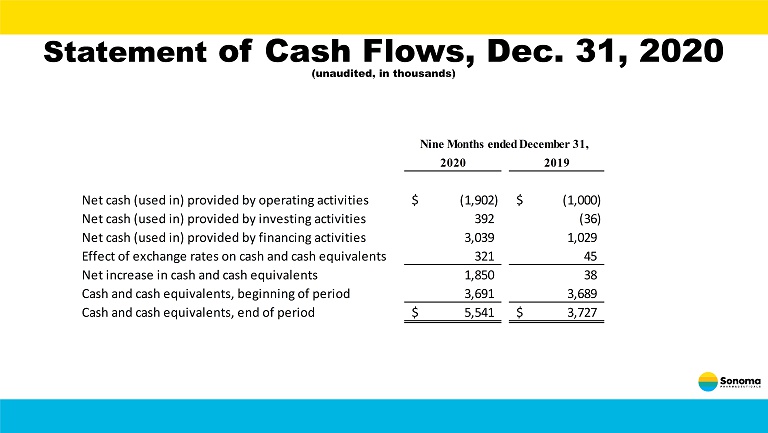
Statement of Cash Flows, Dec. 31, 2020 (unaudited, in thousands) 2020 2019 Net cash (used in) provided by operating activities (1,902)$ (1,000)$ Net cash (used in) provided by investing activities 392 (36) Net cash (used in) provided by financing activities 3,039 1,029 Effect of exchange rates on cash and cash equivalents 321 45 Net increase in cash and cash equivalents 1,850 38 Cash and cash equivalents, beginning of period 3,691 3,689 Cash and cash equivalents, end of period 5,541$ 3,727$ Nine Months ended December 31,
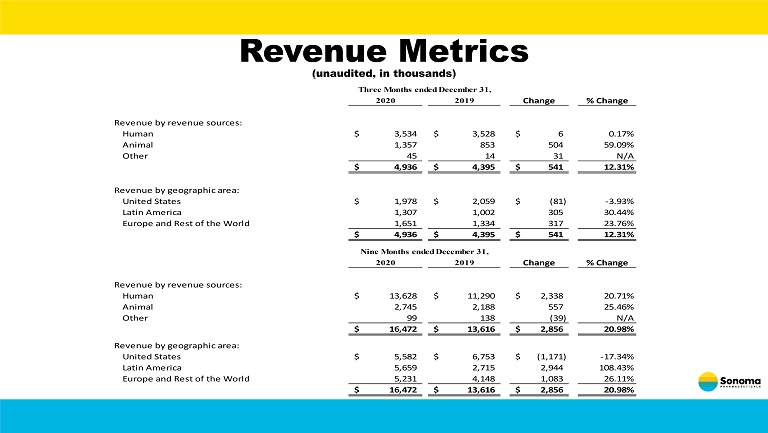
Revenue Metrics (unaudited, in thousands) 2020 2019 Change % Change Revenue by revenue sources: Human 3,534$ 3,528$ 6$ 0.17% Animal 1,357 853 504 59.09% Other 45 14 31 N/A 4,936$ 4,395$ 541$ 12.31% Revenue by geographic area: United States 1,978$ 2,059$ (81)$ -3.93% Latin America 1,307 1,002 305 30.44% Europe and Rest of the World 1,651 1,334 317 23.76% 4,936$ 4,395$ 541$ 12.31% 2020 2019 Change % Change Revenue by revenue sources: Human 13,628$ 11,290$ 2,338$ 20.71% Animal 2,745 2,188 557 25.46% Other 99 138 (39) N/A 16,472$ 13,616$ 2,856$ 20.98% Revenue by geographic area: United States 5,582$ 6,753$ (1,171)$ -17.34% Latin America 5,659 2,715 2,944 108.43% Europe and Rest of the World 5,231 4,148 1,083 26.11% 16,472$ 13,616$ 2,856$ 20.98% Three Months ended December 31, Nine Months ended December 31,

Thank You.